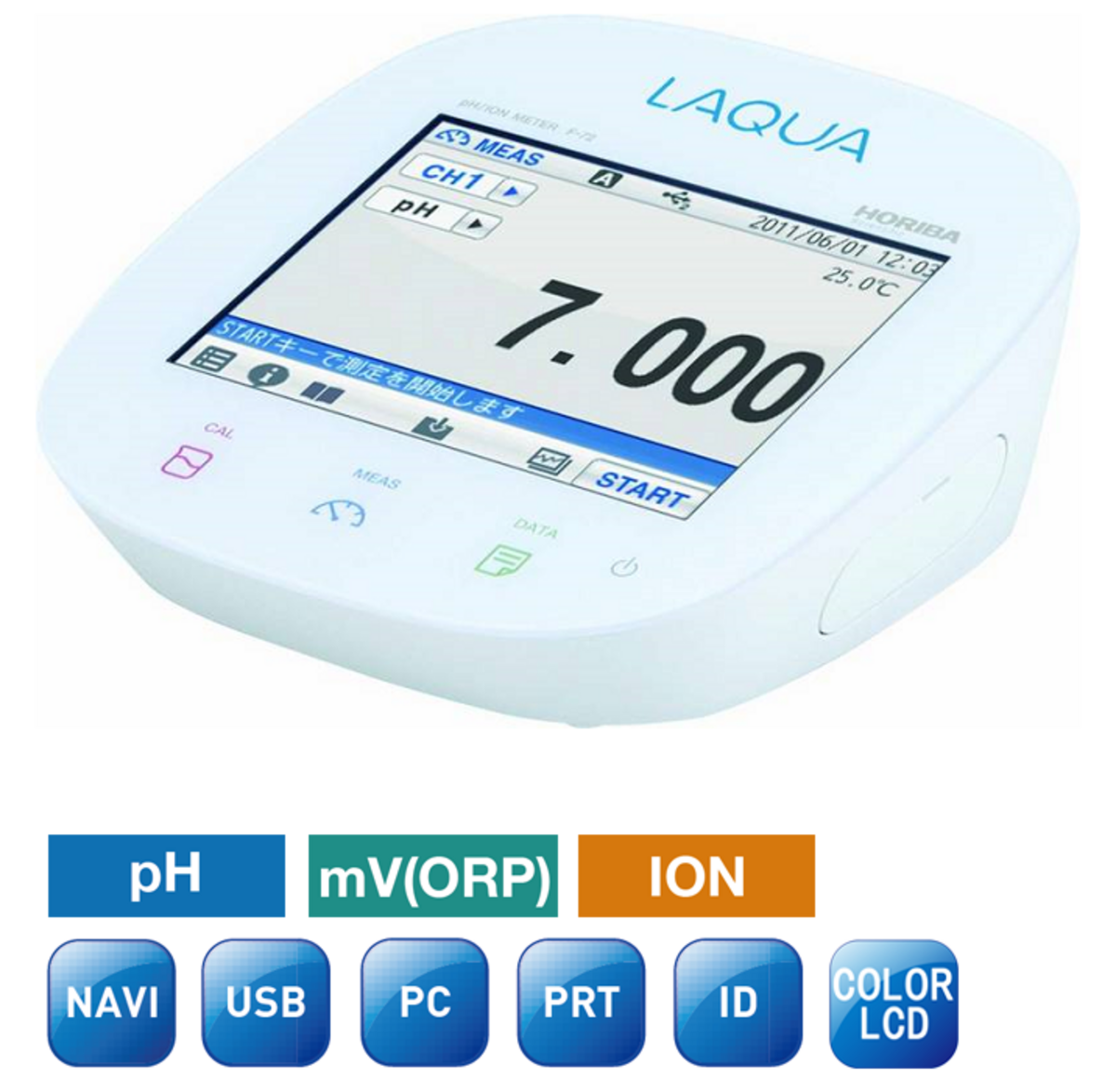
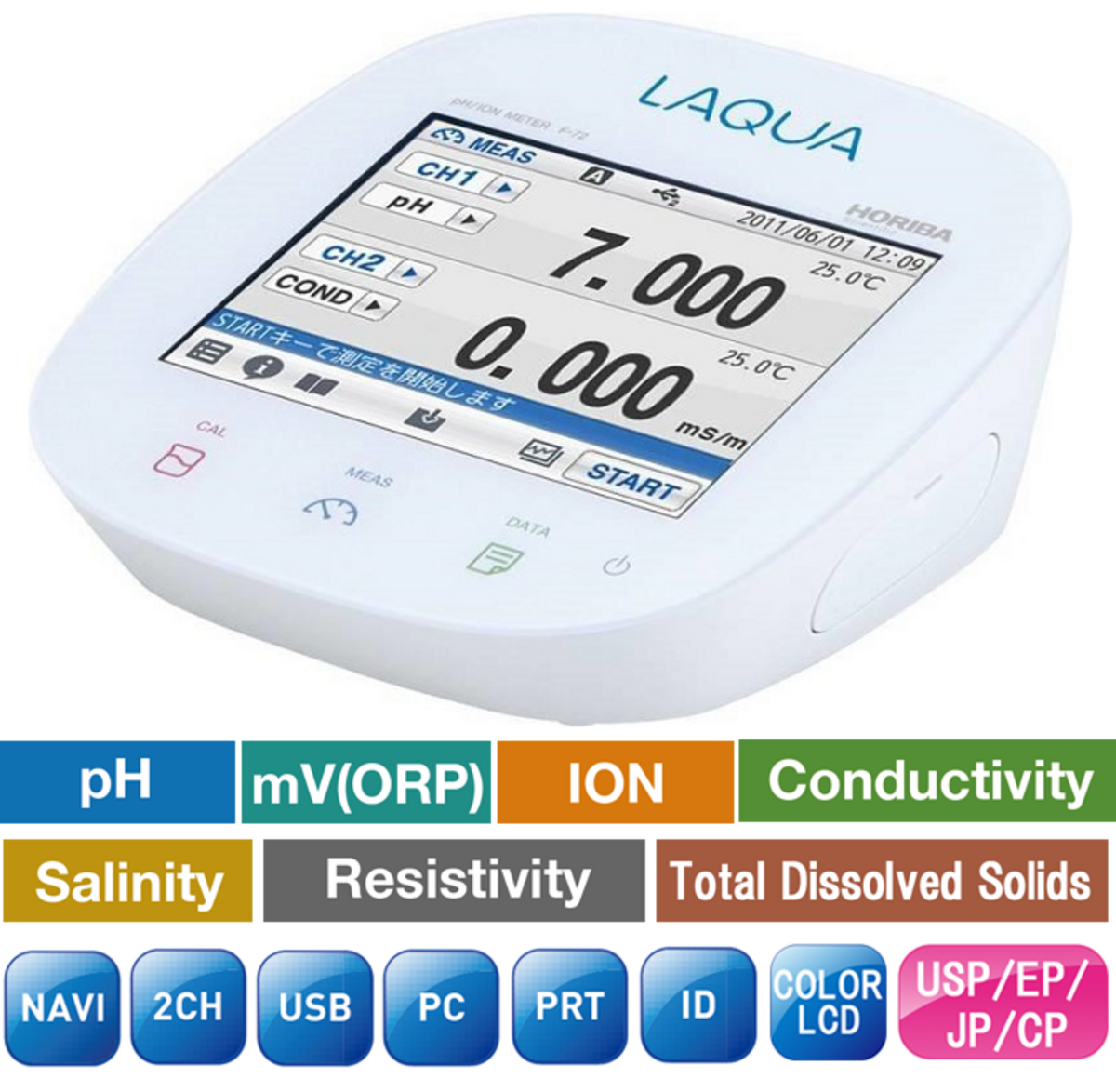
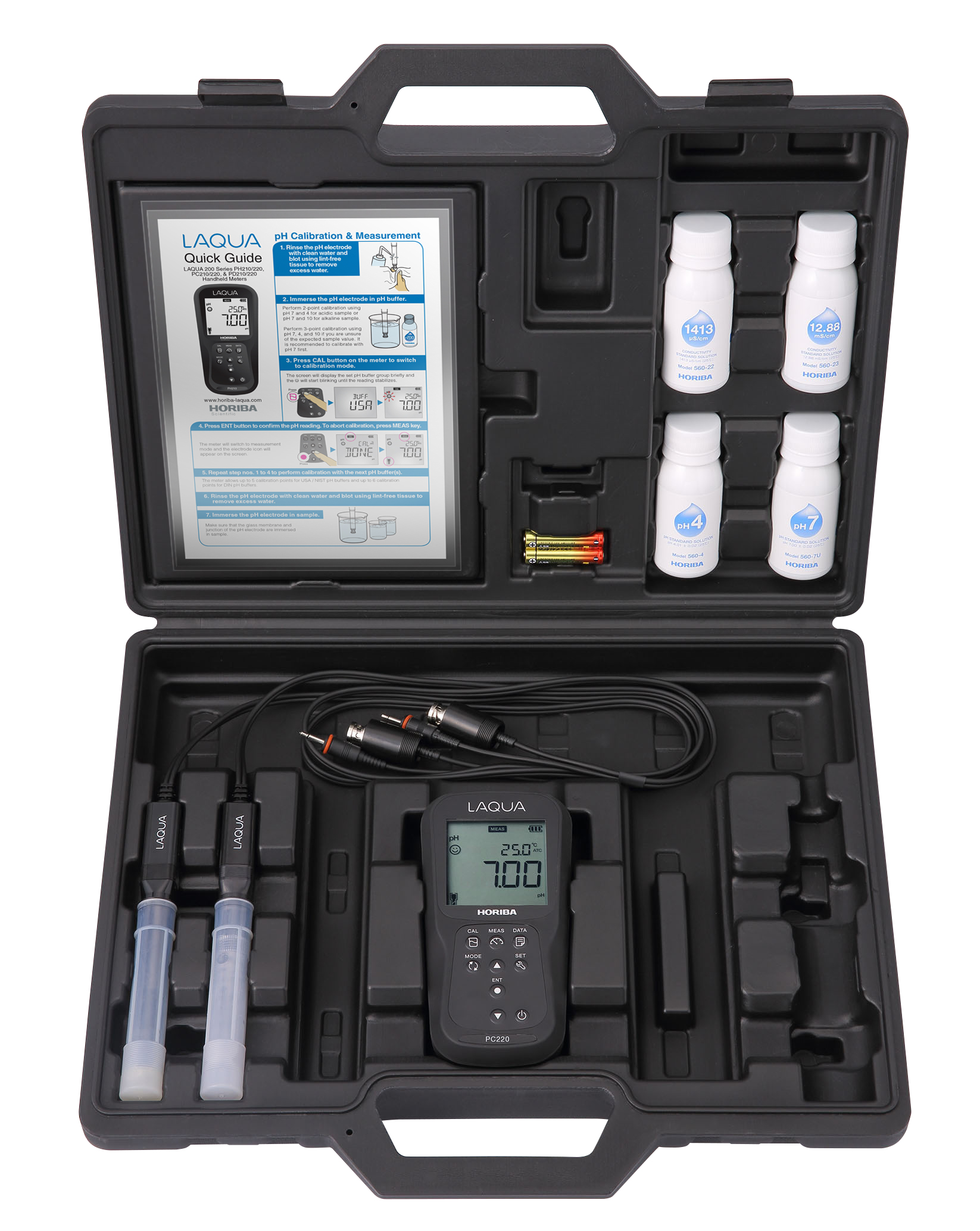
Oxidation-reduction potential (ORP) is an indicator of the oxidizing or reducing ability of a solution and is measured by methods similar to pH.
The ORP is measured using a pH meter with millivoltmeter function in conjunction with a noble metal electrode (platinum or gold) and a reference electrode. The redox potential is affected by temperature and must be measured at a constant temperature (e.g., 25 °C).
In other words, it is the energy level (electric potential) determined by the equilibrium conditions between oxidizing agents (Mz+) and reducing agents (M (z-n) +) coexisting in a solution.
- All Products
-
Parameter
- pH
- ORP
- Conductivity and TDS
- Dissolved oxygen (DO)
- Combined - Multiparameter
- Ammonia (NH3)
- Calcium Ion (Ca2+)
- Chlorid (Cl-)
- Iron (Fe)
- Fluoride (F-)
- Free Chlorine
- Total Chlorine
- Gloss
- Iodine (I)
- Potassium Ion (K+)
- Seawater specific gravity (SSG)
- Sodium Ion (Na+)
- Sodium Chloride (NaCl)
- Nitrate Ion (NO3-)
- Salinity
- Turbidity
- Applications
- Application examples
- Quality
- Brand
- Buffer and calibration solutions
- Spare parts and single components
- Plant analysis - sodium, nitrate, potassium, calcium
- Laboratory Benchtop Meters
- Accessories
Filter products