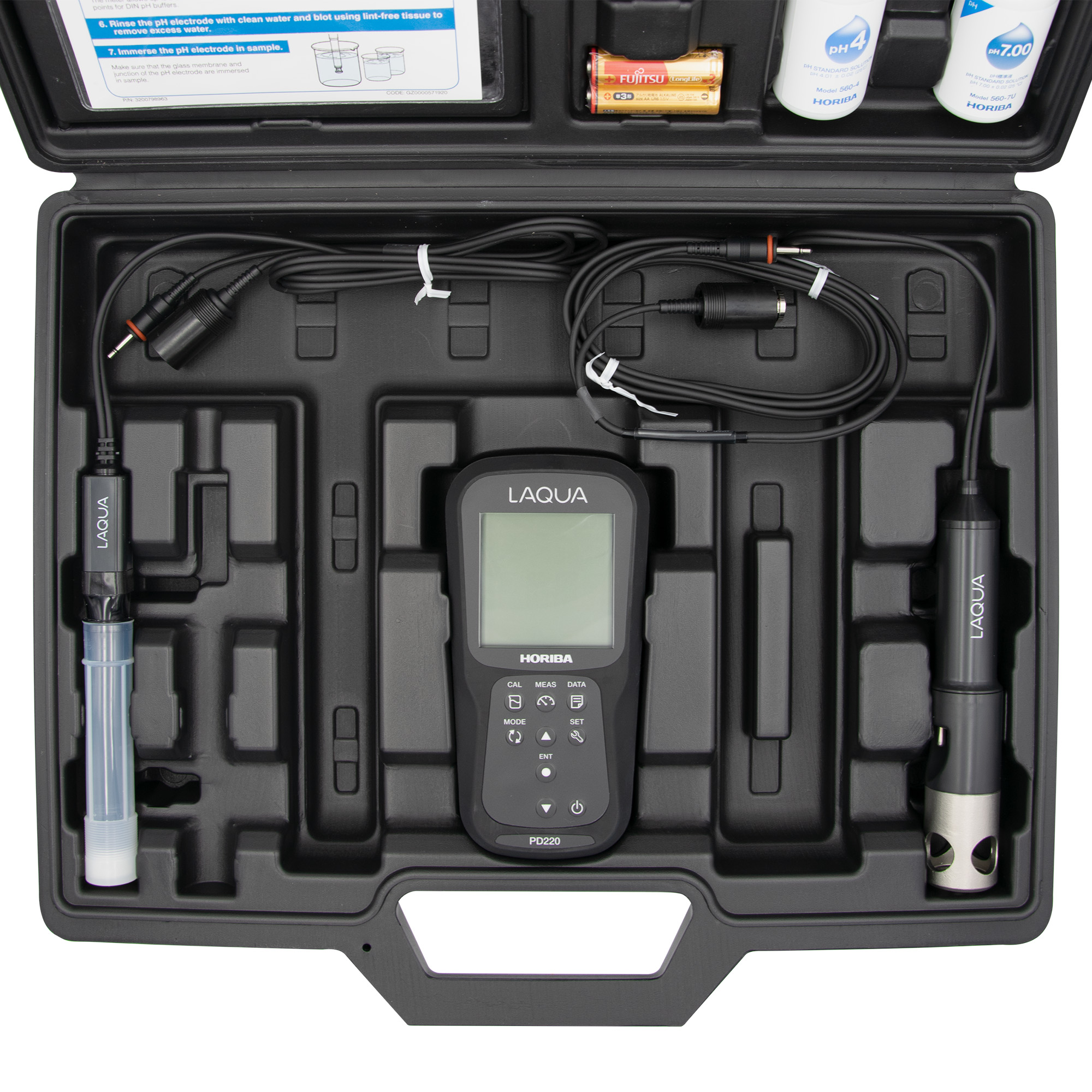
Dissolved oxygen is oxygen dissolved in water and is as important an environmental indicator as pH when measuring the water quality of rivers and lakes. Dissolved oxygen (hereafter abbreviated DO) is oxygen (O2) dissolved in water in nature in proportion to the partial pressure of O2 in the atmosphere. Dissolved oxygen content is expressed as the amount of dissolved O2 per unit volume of water (mg/L).
Fish and other organisms in water live on the oxygen content. DO content is affected by water temperature, salt concentration, atmospheric pressure, and other conditions, and the saturation level decreases as water temperature increases.
In industrial applications, including boilers, make-up water must have low oxygen content to prevent corrosion and scale formation. Monitoring dissolved oxygen levels is critical to ensuring process efficiency, as scale buildup impedes heat transfer. High oxygen content in water improves the taste of drinking water, but increases corrosion in water pipes and transport systems.
- All Products
-
Parameter
- pH
- ORP
- Conductivity and TDS
- Dissolved oxygen (DO)
- Combined - Multiparameter
- Ammonia (NH3)
- Calcium Ion (Ca2+)
- Chlorid (Cl-)
- Iron (Fe)
- Fluoride (F-)
- Free Chlorine
- Total Chlorine
- Gloss
- Iodine (I)
- Potassium Ion (K+)
- Seawater specific gravity (SSG)
- Sodium Ion (Na+)
- Sodium Chloride (NaCl)
- Nitrate Ion (NO3-)
- Salinity
- Turbidity
- Applications
- Application examples
- Quality
- Brand
- Buffer and calibration solutions
- Spare parts and single components
- Plant analysis - sodium, nitrate, potassium, calcium
- Laboratory Benchtop Meters
- Accessories
Filter products